Hazardous Products Regulations (SOR/2015-17)
Full Document:
- HTMLFull Document: Hazardous Products Regulations (Accessibility Buttons available) |
- XMLFull Document: Hazardous Products Regulations [569 KB] |
- PDFFull Document: Hazardous Products Regulations [1196 KB]
Regulations are current to 2024-11-26 and last amended on 2022-12-15. Previous Versions
PART 8Health Hazard Classes (continued)
SUBPART 1Acute Toxicity (continued)
Classification in a Category of the Class (continued)
Classification of Mixtures
Marginal note:Order of provisions
8.1.2 (1) The classification of a mixture as an acute toxicant in a category of this hazard class must proceed in accordance with the order of sections 8.1.3 to 8.1.6.
Marginal note:Concentrations for the purpose of classification
(2) Only ingredients present at concentrations equal to or greater than the concentration limit of 1.0% — w/w for solids, liquids, dusts, mists and vapours and v/v for gases — must be considered for the purpose of classification.
Marginal note:Data available for mixture as a whole
8.1.3 If data of the types referred to in subparagraphs 2.1(a)(i) to (iv) are available for the mixture as a whole, the mixture must be classified as an acute toxicant in accordance with section 8.1.1.
Marginal note:Data available for use of bridging principles
8.1.4 If data are available to enable the characterization of the mixture as an acute toxicant, in accordance with the bridging principles referred to in subsections 2.3(3) to (8), the mixture must be classified in a category of this hazard class in accordance with those subsections.
Marginal note:Data available for all ingredients
8.1.5 If data are available for all ingredients in the mixture, the mixture must be classified as an acute toxicant in accordance with section 8.1.1 using the ATE of the mixture that is determined in respect of each applicable route of exposure by the following formula:
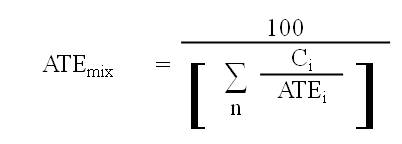
where
- ATEmix
- is the ATE of the mixture determined using this formula;
- Ci
- is the concentration of ingredient i;
- n
- is the number of ingredients and i is running from 1 to n;
- ATEi
- is the ATE of ingredient i, which is either
(a) the LD50 or the LC50 based on or converted to a four-hour exposure period, for i, or
(b) if the LD50 or the LC50 is unavailable, the acute toxicity point estimate established for i in accordance with the table to section 8.1.7; and
- i
- is each ingredient in the mixture with
(a) an ATE within the ranges set out in the applicable table to subsection 8.1.1(3),
(b) an oral or dermal LD50 greater than 2000 mg/kg body weight but less than or equal to 5000 mg/kg body weight, or
(c) an LC50 based on or converted to a four-hour exposure period within a range having an amplitude comparable to the one in paragraph (b).
Marginal note:Data not available for all ingredients
8.1.6 If the ATE is not available for one or more ingredients of the mixture, the mixture must be classified as an acute toxicant in accordance with section 8.1.1 using the ATE of the mixture that is determined in respect of each applicable route of exposure according to the following:
(a) if data permit the ATE to be estimated for each of those ingredients in accordance with established scientific principles, the formula in section 8.1.5 must be used;
(b) if data do not permit the ATE to be estimated for an ingredient in accordance with established scientific principles, and the concentration of the ingredient in the mixture is equal to or greater than the concentration limit of 1.0%, the mixture is classified based only on the ingredients having an ATE, such that
(i) if the total concentration of all ingredients with unknown acute toxicity is less than or equal to 10.0% of the mixture, the formula in section 8.1.5 must be used, or
(ii) if the total concentration of all ingredients with unknown acute toxicity is greater than 10.0% of the mixture, the following formula must be used:
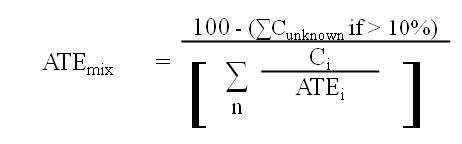
where
- ATEmix
- is the ATE of the mixture determined using this formula,
- Ci
- is the concentration of ingredient i,
- Cunknown
- is the concentration of ingredients i with unknown ATE values,
- n
- is the number of ingredients and i is running from 1 to n,
- ATEi
- is the ATE of ingredient i, which is either
(a) the LD50 or the LC50 based on or converted to a four-hour exposure period, for i, or
(b) if the LD50 or the LC50 is unavailable, the acute toxicity point estimate established for i in accordance with the table to section 8.1.7, and
- i
- is each ingredient in the mixture with
(a) an ATE within the ranges set out in the applicable table to subsection 8.1.1(3),
(b) an oral or dermal LD50 greater than 2000 mg/kg body weight but less than or equal to 5000 mg/kg body weight, or
(c) an LC50 based on or converted to a four-hour exposure period within a range having an amplitude comparable to the one in paragraph (b).
Marginal note:Conversion from range to point estimate
8.1.7 (1) If a formula in section 8.1.5 or 8.1.6 is used, an acute toxicity point estimate must be determined, in accordance with the table to subsection (2), for each ingredient for which only that ingredient’s classification category or experimentally obtained acute toxicity range is available.
Marginal note:More than one range
(2) If the experimentally obtained acute toxicity range for an ingredient does not fall entirely within any of the ranges set out in column 2 of the following table, the converted acute toxicity point estimate for that ingredient for the purposes of column 3 is the lowest value of the experimentally obtained acute toxicity range.
TABLE
Column 1 Column 2 Column 3 Item Exposure Routes Classification Category and Associated Experimentally Obtained Acute Toxicity Range Minimum and Maximum Values Converted Acute Toxicity Point Estimate 1 Oral (mg/kg body weight) - 0 < Category 1 ≤ 5
- 5 < Category 2 ≤ 50
- 50 < Category 3 ≤ 300
- 300 < Category 4 ≤ 2000
- 0.5
- 5
- 100
- 500
2 Dermal (mg/kg body weight) - 0 < Category 1 ≤ 50
- 50 < Category 2 ≤ 200
- 200 < Category 3 ≤ 1000
- 1000 < Category 4 ≤ 2000
- 5
- 50
- 300
- 1100
3 Inhalation (gases) (ppmV) - 0 < Category 1 ≤ 100
- 100 < Category 2 ≤ 500
- 500 < Category 3 ≤ 2500
- 2500 < Category 4 ≤ 20 000
- 10
- 100
- 700
- 4500
4 Inhalation (vapours) (mg/l) - 0 < Category 1 ≤ 0.5
- 0.5 < Category 2 ≤ 2.0
- 2.0 < Category 3 ≤ 10.0
- 10.0 < Category 4 ≤ 20.0
- 0.05
- 0.5
- 3
- 11
5 Inhalation (dust/mist) (mg/l) - 0 < Category 1 ≤ 0.05
- 0.05 < Category 2 ≤ 0.5
- 0.5 < Category 3 ≤ 1.0
- 1.0 < Category 4 ≤ 5.0
- 0.005
- 0.05
- 0.5
- 1.5
SUBPART 2Skin Corrosion/Irritation
Definitions
Marginal note:Definitions
8.2 The following definitions apply in this Subpart.
- skin corrosion
skin corrosion means the production of irreversible damage to the skin, namely, visible necrosis through the epidermis and into the dermis, occurring after exposure to a mixture or substance, and includes ulcers, bleeding, bloody scabs and, within a 14-day observation period, discoloration due to blanching of the skin, complete areas of alopecia, and scars. (corrosion cutanée)
- skin-corrosive
skin-corrosive means, in relation to a mixture or substance, liable to cause skin corrosion. (corrosif pour la peau)
- skin-irritant
skin-irritant means, in relation to a mixture or substance, liable to cause skin irritation. (irritant pour la peau)
- skin irritation
skin irritation means the production of reversible damage to the skin occurring after exposure to a mixture or substance. (irritation cutanée)
Classification in a Category or Subcategory of the Class
Classification of Substances
Marginal note:Order of provisions
8.2.1 The classification of a skin-corrosive substance or a skin-irritant substance in a category or subcategory of this hazard class must proceed in accordance with the order of sections 8.2.2 to 8.2.7, unless, after applying subsections 8.2.2(1) to (3), the substance is not classified further to subsection 8.2.2(4).
- Date modified: